Byrne, Robert, Coleman, Simon, Răduţă, Ana Maria, Fraser, Kevin J.  ORCID: 0000-0002-9718-5405, MacFarlane, Douglas R. and Diamond, Dermot
ORCID: 0000-0002-9718-5405, MacFarlane, Douglas R. and Diamond, Dermot  ORCID: 0000-0003-2944-4839
(2009)
Photochromism of nitrobenzospiropyran in phosphonium based ionic liquids.
Physical Chemistry Chemical Physics, 11
(33).
pp. 7286-7291.
ISSN 1463-9084
ORCID: 0000-0003-2944-4839
(2009)
Photochromism of nitrobenzospiropyran in phosphonium based ionic liquids.
Physical Chemistry Chemical Physics, 11
(33).
pp. 7286-7291.
ISSN 1463-9084
Abstract
The photo-, thermo- and solvatochromic properties of 2,3-dihydro-1,3,3-trimethyl-6-nitrospiro[1-benzopyran-2,2-1H-indole] (BSP) and its photo-induced merocyanine isomer (MC) were investigated in phosphonium based ILs by UV-vis absorption spectroscopy. It was found that the kinetics and thermodynamics of the BSP MC equilibrium were sensitive to the nature of the anion. The MC max shifted from 560 nm to 578 nm when in solutions of [P1,4,4,4][tos] and [P6,6,6,14][dca], respectively. The BSP isomer was highly favoured at equilibrium in the ILs studied; Ke values observed were similar to non-polar solvents such as dichloromethane. The thermal relaxation of MC in all ILs is first order, and in comparison with aprotic polar solvents possessing comparable polarity (such as acetonitrile). Thermal relaxation rates of MC were monitored over a range of temperatures; at 293 K rates varied from 5.19 to 25.03 × 10-4 s-1. A non-linear relationship between Ke and k was observed; this contradicts what is expected for BSP in molecular solvents and suggests the isomers exhibit different molecular/solvation environments. The energetics of the thermal relaxation of MC in ILs were observed; activation energies ranged from 71 to 90 kJ mol-1 and all ILs exhibit negative activation entropies ranging between -72 and -8.2 J K-1 mol-1. A linear relationship between activation energy and entropy was observed.
Metadata
| Item Type: | Article (Published) |
|---|---|
| Refereed: | Yes |
| Subjects: | Physical Sciences > Photochemistry Physical Sciences > Chemistry |
| DCU Faculties and Centres: | DCU Faculties and Schools > Faculty of Science and Health > School of Chemical Sciences Research Initiatives and Centres > Biomedical Diagnostics Institute (BDI) Research Initiatives and Centres > CLARITY: The Centre for Sensor Web Technologies Research Initiatives and Centres > National Centre for Sensor Research (NCSR) |
| Publisher: | Royal Society of Chemistry |
| Official URL: | http://dx.doi.org/10.1039/b903772a |
| Use License: | This item is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 3.0 License. View License |
| Funders: | Science Foundation Ireland |
| ID Code: | 4673 |
| Deposited On: | 10 Jul 2009 10:27 by Robert Byrne . Last Modified 19 Sep 2018 08:53 |
Documents
Full text available as:
Preview |
PDF
- Requires a PDF viewer such as GSview, Xpdf or Adobe Acrobat Reader
799kB |
![[thumbnail of b903772a-ga.gif]](https://doras.dcu.ie/4673/3.hassmallThumbnailVersion/b903772a-ga.gif) 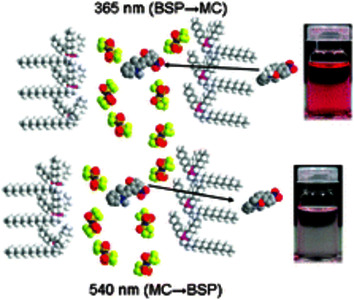 Preview |
Image (GIF)
18kB |
Downloads
Downloads
Downloads per month over past year
Archive Staff Only: edit this record